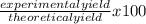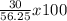Chemistry, 20.11.2019 23:31 jacobs5919
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + cl2 → c6h5cl + hcl , what is the percent yield of c6h5cl? 1. 50.0% 2. 53.4% 3. 69.4% 4. 13.2% 5. 76.9%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + c...
Questions


Health, 05.02.2020 01:54

History, 05.02.2020 01:54



Mathematics, 05.02.2020 01:54



History, 05.02.2020 01:54




Biology, 05.02.2020 01:54




English, 05.02.2020 01:54



History, 05.02.2020 01:54