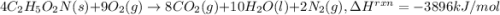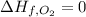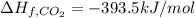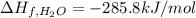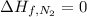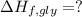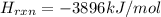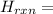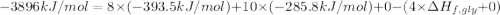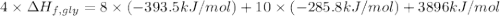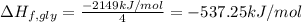Chemistry, 20.11.2019 21:31 clickbaitdxl
Glycine, c2h5o2n, is important for biological energy. the combustion reaction of glycine is described by the following thermochemical equation. 4c2h5o2n(s) + 9o2(g) → 8co2(g) + 10h2o(l) + 2n2(g) δh°rxn = –3896 kj/molwhat is the standard enthalpy of formation of solid glycine? –51.90 kj/mol–527.5 kj/mol–974.0 kj/mol–1502 kj/mol–2476 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Glycine, c2h5o2n, is important for biological energy. the combustion reaction of glycine is describe...
Questions


Mathematics, 15.07.2019 04:00

Health, 15.07.2019 04:00

Mathematics, 15.07.2019 04:00




Mathematics, 15.07.2019 04:00



Mathematics, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00


English, 15.07.2019 04:00

Chemistry, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00

English, 15.07.2019 04:00