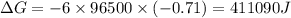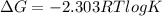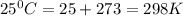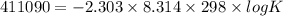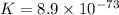Chemistry, 20.11.2019 20:31 pennyluvsu13
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following balanced redox reaction at 25°c. 2 al(s) + 3 mg2+(aq) → 2 al3+(aq) + 3 mg(s) a) 1.1 × 1072 b) 8.9 × 10-73 c) 1.1 × 10-72 d) 1.0 × 1024 e) 4.6 × 1031

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following b...
Questions

Arts, 14.02.2020 09:06



Mathematics, 14.02.2020 09:07




Mathematics, 14.02.2020 09:09

Biology, 14.02.2020 09:09





History, 14.02.2020 09:14





Mathematics, 14.02.2020 09:14

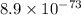
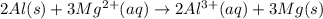
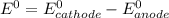
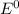 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Mg^{2+}/Mg]}= -2.37V](/tpl/images/0383/2083/24fc1.png)
![E^0_{[Al^{3+}/Al]}=-1.66V](/tpl/images/0383/2083/0867c.png)
![E^0=E^0_{[Mg^{2+}/Mg]}- E^0_{[Al^{3+}/Al]}](/tpl/images/0383/2083/0b323.png)
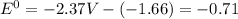

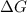 = gibbs free energy
= gibbs free energy