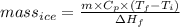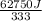Chemistry, 20.11.2019 20:31 jdsfdujfi1598
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitcher. if the tea is initially at 30.0°c and the ice cubes are initially at 0.0°c, how many grams of ice will still be present when the contents of the pitcher reach a final temperature? the tea is mostly water, so assume that it has the same density (1.0 g/ml), molar mass, heat capacity (75.3 j/k/mol), and heat of fusion (6.01 kj/mol) as pure water. the heat capacity of ice is 37.7 j/k/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
4nh3+5o2--> 4no+6h20what is the total number of moles of h2o produced when 12 mole of nh3 is completely consumed?
Answers: 3

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitche...
Questions



Computers and Technology, 14.12.2019 10:31

Mathematics, 14.12.2019 10:31

Mathematics, 14.12.2019 10:31

Computers and Technology, 14.12.2019 10:31

Business, 14.12.2019 10:31



Mathematics, 14.12.2019 10:31

Computers and Technology, 14.12.2019 10:31

Computers and Technology, 14.12.2019 10:31


Spanish, 14.12.2019 10:31



Mathematics, 14.12.2019 10:31

Health, 14.12.2019 10:31


Mathematics, 14.12.2019 10:31

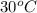 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K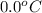 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K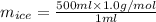
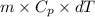
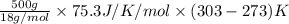
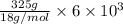
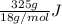 heat but we have 40774.95 J.
heat but we have 40774.95 J.