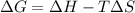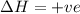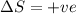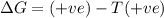Chemistry, 20.11.2019 20:31 Legoman29305
Consider a reaction that has a positive δh and a positive δs. which of the following statements is true? a) this reaction will be spontaneous only at high temperatures. b) this reaction will be spontaneous at all temperatures. c) this reaction will be nonspontaneous at all temperatures. d) this reaction will be nonspontaneous only at high temperatures. e) it is not possible to determine without more information.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Consider a reaction that has a positive δh and a positive δs. which of the following statements is t...
Questions



Business, 10.03.2020 09:03



Mathematics, 10.03.2020 09:03

Mathematics, 10.03.2020 09:03




Computers and Technology, 10.03.2020 09:03


Biology, 10.03.2020 09:03





Mathematics, 10.03.2020 09:03

Biology, 10.03.2020 09:03

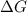 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous