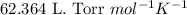Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
The air in a bicycle tire is bubbled through water and collected at 25 ∘c. if the total volume of ga...
Questions


History, 04.04.2021 14:00


Law, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00



Mathematics, 04.04.2021 14:00

Mathematics, 04.04.2021 14:00


History, 04.04.2021 14:00

Chemistry, 04.04.2021 14:00


Mathematics, 04.04.2021 14:00


Mathematics, 04.04.2021 14:00



Biology, 04.04.2021 14:00

SAT, 04.04.2021 14:00

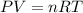
![25^oC=[25+273]K=298K](/tpl/images/0383/2623/df1f6.png)