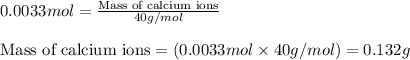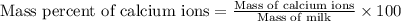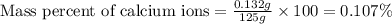The amount ofcalcium present in milk can be determined by adding oxalate to asample and measuring the massof calcium oxalate precipitated. what is the mass percent ofcalcium if 0.429 g of calcium oxalate forms in a125-g sample of milk when excess aqueous sodium oxalate isadded? na2c2o4(aq) +ca2+(aq) → cac2o4(s) +2na+(aq) a. 0.107% b. 0.202% c. 0.343% d. 1.10% e.1.37%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2
You know the right answer?
The amount ofcalcium present in milk can be determined by adding oxalate to asample and measuring th...
Questions

Mathematics, 01.11.2019 11:31

History, 01.11.2019 11:31

History, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31


Mathematics, 01.11.2019 11:31


English, 01.11.2019 11:31


Business, 01.11.2019 11:31


Social Studies, 01.11.2019 11:31






Mathematics, 01.11.2019 11:31

History, 01.11.2019 11:31

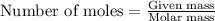 .....(1)
.....(1)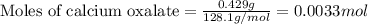
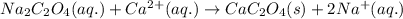
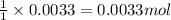 of calcium ions
of calcium ions