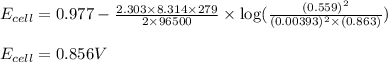Chemistry, 20.11.2019 17:31 hoosierkid5633
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (aq) + 2 h+ + 2 e- → 2 h2o eo = 1.78 v what will the potential of this cell be when [ag+] = 0.559 m, [h+] = 0.00393 m, and [h2o2] = 0.863 m?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (...
Questions






Social Studies, 20.07.2019 00:30

History, 20.07.2019 00:30





Social Studies, 20.07.2019 00:30




Social Studies, 20.07.2019 00:30

Biology, 20.07.2019 00:30

Mathematics, 20.07.2019 00:30

Biology, 20.07.2019 00:30

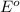 potential will always get reduced.
potential will always get reduced.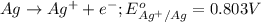 ( × 2)
( × 2)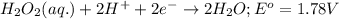
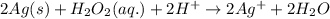
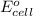 of the reaction, we use the equation:
of the reaction, we use the equation: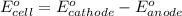
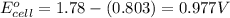
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ag^{+}]^2}{[H^{+}]^2[H_2O_2]}](/tpl/images/0383/1046/4a00d.png)
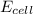 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Ag^{+}]=0.559M](/tpl/images/0383/1046/b53ec.png)
![[H^{+}]=0.00393M](/tpl/images/0383/1046/74117.png)
![[H_2O_2]=0.863M](/tpl/images/0383/1046/04f5b.png)