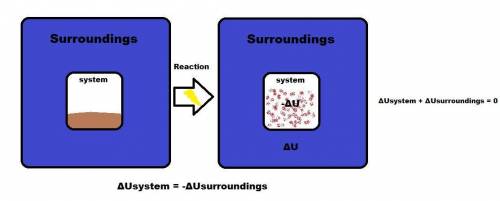
The internal energy can be increased by
(a) transferring heat from the surroundings to the sy...

Chemistry, 20.11.2019 02:31 fjjjjczar8890
The internal energy can be increased by
(a) transferring heat from the surroundings to the system.
(b) transferring heat from the system to the surroundings.
(c) doing work on the system.
a) a only.
b) b only.
c) c only.
d) a and c.
e) b and c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1


Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
Questions


English, 13.10.2019 09:50

History, 13.10.2019 09:50






Mathematics, 13.10.2019 09:50


Biology, 13.10.2019 09:50

Chemistry, 13.10.2019 09:50


Mathematics, 13.10.2019 09:50


Physics, 13.10.2019 09:50


Mathematics, 13.10.2019 09:50

English, 13.10.2019 09:50