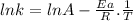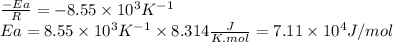The rate constant of a reaction is measured at different temperatures. a plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of −8.55×103 k−1. what is the activation energy (ea) for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
The rate constant of a reaction is measured at different temperatures. a plot of the natural log of...
Questions


Mathematics, 10.12.2020 17:30



Chemistry, 10.12.2020 17:30



Mathematics, 10.12.2020 17:30




Health, 10.12.2020 17:30

Mathematics, 10.12.2020 17:30

Mathematics, 10.12.2020 17:30

Biology, 10.12.2020 17:30



Chemistry, 10.12.2020 17:30