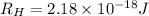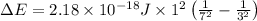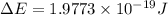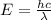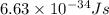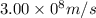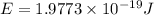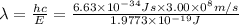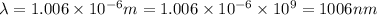Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
Given that rh= 2.18 x 10⁻¹⁸j, 1 nm = 1 x 10⁻⁹m, h = 6.63 x 10⁻³⁴j·s, and c = 3.00 x 10⁸m/s:
c...
c...
Questions

Mathematics, 11.01.2021 23:30

Mathematics, 11.01.2021 23:30



Mathematics, 11.01.2021 23:30

History, 11.01.2021 23:30

Mathematics, 11.01.2021 23:30

History, 11.01.2021 23:30




Mathematics, 11.01.2021 23:30

Mathematics, 11.01.2021 23:30


Mathematics, 11.01.2021 23:30

Mathematics, 11.01.2021 23:30



Arts, 11.01.2021 23:30

Mathematics, 11.01.2021 23:30

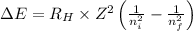
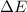 = Energy difference
= Energy difference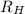 = Rydberg's Constant
= Rydberg's Constant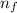 = Final energy level
= Final energy level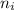 = Initial energy level
= Initial energy level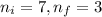 , Z = 1
, Z = 1