Chemistry, 19.11.2019 21:31 keving4three
Consider the reaction ch4 + 2 o2 → co2 + 2 h2o exactly 14.5 moles of co2 are produced upon reaction of 34.6 moles of o2 and how many moles of ch4?
1. 65.2 moles
2. 14.5 moles
3. 34.5 moles
4. 29.0 moles
5. 16.3 moles
6. 17.3 moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Consider the reaction ch4 + 2 o2 → co2 + 2 h2o exactly 14.5 moles of co2 are produced upon reaction...
Questions


Mathematics, 22.10.2019 02:00




Mathematics, 22.10.2019 02:00

Mathematics, 22.10.2019 02:00

Mathematics, 22.10.2019 02:00





Chemistry, 22.10.2019 02:00

History, 22.10.2019 02:00


Mathematics, 22.10.2019 02:00

Mathematics, 22.10.2019 02:00


Mathematics, 22.10.2019 02:00

Chemistry, 22.10.2019 02:00

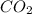 formation is:
formation is: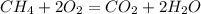
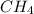 reacted to form this quantity:
reacted to form this quantity: