

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1

Chemistry, 23.06.2019 18:20
In a chemical reaction, the number of moles of the reactants a. should never be equal to the number of moles of the products. b. may or may not be equal to the number of moles of the products. c. depends on the amount of product that is formed. d. should always be equal to the number of moles of the products.
Answers: 3
You know the right answer?
Consider the complete combustion of glucose (c6h12o6) with o2 and calculate the moles of co2 produce...
Questions


English, 17.05.2021 21:40




Physics, 17.05.2021 21:40





Mathematics, 17.05.2021 21:40

History, 17.05.2021 21:40


Mathematics, 17.05.2021 21:50

Mathematics, 17.05.2021 21:50

Mathematics, 17.05.2021 21:50

Mathematics, 17.05.2021 21:50



Mathematics, 17.05.2021 21:50

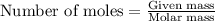
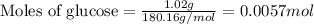
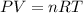
![37^oC=[37+273]K=310K](/tpl/images/0381/5720/20b22.png)
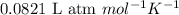
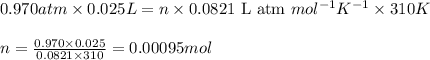
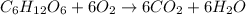
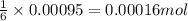 of glucose
of glucose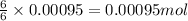 of carbon dioxide
of carbon dioxide

