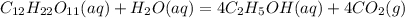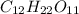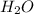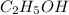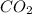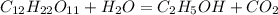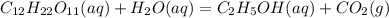Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Write a balanced chemical equation for the fermentation of sucrose (c12h22o11) by yeasts in which th...
Questions

Mathematics, 27.10.2020 20:40

SAT, 27.10.2020 20:40


History, 27.10.2020 20:40


History, 27.10.2020 20:40

Mathematics, 27.10.2020 20:40

Mathematics, 27.10.2020 20:40

History, 27.10.2020 20:40

Mathematics, 27.10.2020 20:40

History, 27.10.2020 20:40

Mathematics, 27.10.2020 20:40

Mathematics, 27.10.2020 20:40

Geography, 27.10.2020 20:40

Computers and Technology, 27.10.2020 20:40


English, 27.10.2020 20:40


Mathematics, 27.10.2020 20:40

Social Studies, 27.10.2020 20:40