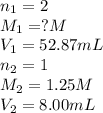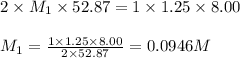Chemistry, 19.11.2019 17:31 zeesharpe05
8.00ml of 1.25m lithiukm hydroxide is reacted with sulfuric acid. it is found that 52.87ml of the sulfuric acid is required to completely neutralize the lithium hydroxide. what is the approximate molarity of sulfuric acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
8.00ml of 1.25m lithiukm hydroxide is reacted with sulfuric acid. it is found that 52.87ml of the su...
Questions

Spanish, 09.11.2020 04:50

Chemistry, 09.11.2020 04:50



Mathematics, 09.11.2020 04:50

Chemistry, 09.11.2020 04:50

Mathematics, 09.11.2020 04:50

Mathematics, 09.11.2020 04:50

Mathematics, 09.11.2020 04:50



Biology, 09.11.2020 04:50

Mathematics, 09.11.2020 04:50

World Languages, 09.11.2020 04:50

English, 09.11.2020 04:50





Mathematics, 09.11.2020 04:50

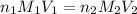
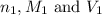 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 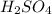
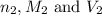 are the n-factor, molarity and volume of base which is LiOH
are the n-factor, molarity and volume of base which is LiOH