Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
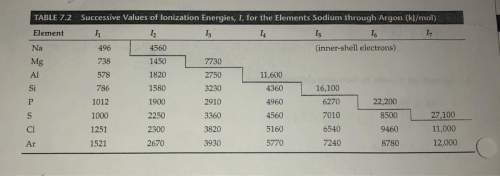
Where would the largest jump in ionization energies be for oxygen? (with the loss of how many elect...
Questions

Chemistry, 31.08.2020 14:01


English, 31.08.2020 14:01

Biology, 31.08.2020 14:01

Computers and Technology, 31.08.2020 14:01

Mathematics, 31.08.2020 14:01


Mathematics, 31.08.2020 14:01

Mathematics, 31.08.2020 14:01

History, 31.08.2020 14:01

Physics, 31.08.2020 14:01

English, 31.08.2020 14:01



History, 31.08.2020 14:01

Computers and Technology, 31.08.2020 14:01

Chemistry, 31.08.2020 14:01


Computers and Technology, 31.08.2020 14:01

History, 31.08.2020 14:01