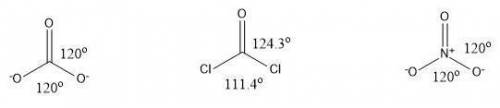
Consider the following molecules with trigonal planar geometry. carbonate (co32−) and nitrate (no3−) both exhibit resonance, whereas phosgene (cocl2) does not. given the information in the transition, and ignoring the simulation, predict the bond angles for each of the molecules in accordance to whether or not they exhibit resonance. drag the appropriate labels to their respective targets. view available hint(s)\

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2


Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
Consider the following molecules with trigonal planar geometry. carbonate (co32−) and nitrate (no3−)...
Questions



Mathematics, 19.04.2020 04:14


Chemistry, 19.04.2020 04:14


Health, 19.04.2020 04:14

Physics, 19.04.2020 04:14


Social Studies, 19.04.2020 04:15

Mathematics, 19.04.2020 04:15



Mathematics, 19.04.2020 04:16

Mathematics, 19.04.2020 04:16



Mathematics, 19.04.2020 04:16

Mathematics, 19.04.2020 04:16

Mathematics, 19.04.2020 04:16