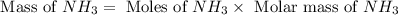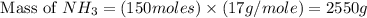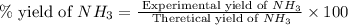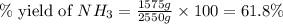Chemistry, 19.11.2019 06:31 jlbradley429
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with nitrogen, 1575 g of ammonia were produced. what is the percent yield of this reaction?
30.8%
61.8%
20.7%
41.5%
more information is needed to solve this problem.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with...
Questions


English, 04.10.2021 21:40




English, 04.10.2021 21:40

SAT, 04.10.2021 21:40


History, 04.10.2021 21:40

History, 04.10.2021 21:40




Mathematics, 04.10.2021 21:50

Mathematics, 04.10.2021 21:50




English, 04.10.2021 21:50

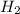 = 450 g
= 450 g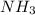 = 17 g/mole
= 17 g/mole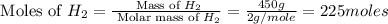
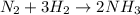
 moles of
moles of