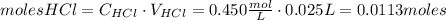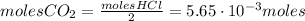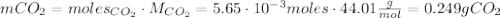Chemistry, 19.11.2019 06:31 caromaybelline71
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the following equation: caco3(s) 2hcl(aq)⟶co2(g) h2o(l) cacl2(aq) a typical antacid contains caco3. if such an antacid is added to 25.0 ml of a solution that is 0.450 m in hcl, how many grams of co2 gas are produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the followi...
Questions



English, 06.04.2020 23:32







Mathematics, 06.04.2020 23:32



Mathematics, 06.04.2020 23:32

Mathematics, 06.04.2020 23:32

Mathematics, 06.04.2020 23:32



Biology, 06.04.2020 23:32