
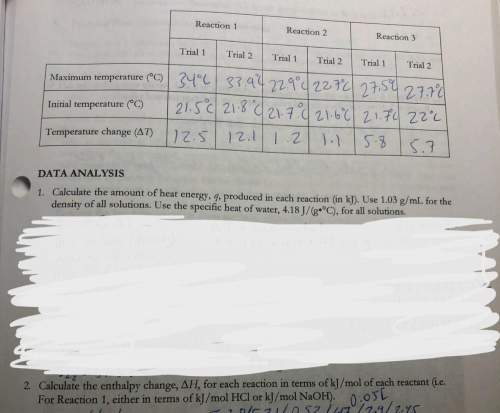
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for reaction three.
4. use hess’s law, and the accepted values of change of h in the pre-lab exercise to calculate the change in h for reaction 3. how does the accepted value compare to your experimental value?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for rea...
Questions

History, 20.07.2019 11:40

French, 20.07.2019 11:40


English, 20.07.2019 11:40

History, 20.07.2019 11:40


Mathematics, 20.07.2019 11:40


Mathematics, 20.07.2019 11:40

Biology, 20.07.2019 11:40

Physics, 20.07.2019 11:40

English, 20.07.2019 11:40



History, 20.07.2019 11:40





Business, 20.07.2019 11:40



