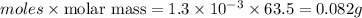Chemistry, 19.11.2019 05:31 nayellisoto15
Acopper cycle experiment takes copper atoms through reactions that produce copper compounds and complexes one after the other, finally producing elemental copper. copper atoms are conserved throughout the process. given that a student begins with 9.29 ml of a 0.14 m cu(no3)2 solution, how much copper should be isolated at the end of the cycle?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Acopper cycle experiment takes copper atoms through reactions that produce copper compounds and comp...
Questions



Mathematics, 15.12.2021 18:00


Physics, 15.12.2021 18:00


Biology, 15.12.2021 18:00


Mathematics, 15.12.2021 18:00


Mathematics, 15.12.2021 18:00

Mathematics, 15.12.2021 18:00

History, 15.12.2021 18:00




Mathematics, 15.12.2021 18:00

Mathematics, 15.12.2021 18:00

English, 15.12.2021 18:00

Chemistry, 15.12.2021 18:10

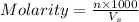
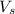 = volume of solution in ml = 9.29 ml
= volume of solution in ml = 9.29 ml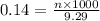
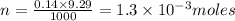
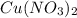 contains 1 mole of copper
contains 1 mole of copper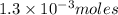 moles of
moles of 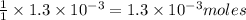 of copper
of copper