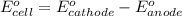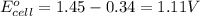Chemistry, 19.11.2019 03:31 mahadharun
Calculate the standard cell emf for the reaction: clo3−(aq)+3cu(s)+6h+(aq)→cl−(aq)+3c u2+(aq)+3h2o(l) pt is used as an inert electrode in contact with the clo3− and cl−. calculate the standard emf using data in appendix e and given the following: clo3−(aq)+6h+(aq)+6e−→cl−(aq)+3h2o( l); e∘=1.45 v express the emf to three significant figures with the appropriate units. v

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Calculate the standard cell emf for the reaction: clo3−(aq)+3cu(s)+6h+(aq)→cl−(aq)+3c u2+(aq)+3h2o(...
Questions



Mathematics, 25.01.2021 19:30

Mathematics, 25.01.2021 19:30

Spanish, 25.01.2021 19:30





Biology, 25.01.2021 19:30

History, 25.01.2021 19:30

Mathematics, 25.01.2021 19:30

History, 25.01.2021 19:30


Mathematics, 25.01.2021 19:30

Mathematics, 25.01.2021 19:30



Mathematics, 25.01.2021 19:30

Chemistry, 25.01.2021 19:30

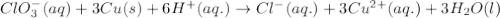
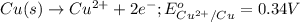 ( × 3)
( × 3)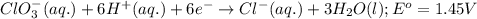
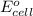 of the reaction, we use the equation:
of the reaction, we use the equation: