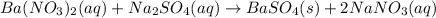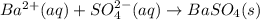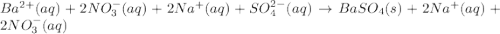Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined. upon mixing, a thick, milky white precipitate formed. after centrifugation, the solution above the precipitate was found to be clear and colorless. based on these observations, determine if a reaction occurred. if so, write the balanced chemical equation and net ionic equation for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined....
Questions





Mathematics, 03.10.2021 19:40




Mathematics, 03.10.2021 19:40



Mathematics, 03.10.2021 19:40




Mathematics, 03.10.2021 19:40

History, 03.10.2021 19:40