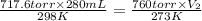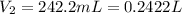Chemistry, 19.11.2019 03:31 carapiasebas
Atrial of this decomposition experiment, using different quantities of reactants than those listed in the question above produced the following data: volume of o2 produced at room conditions 280 ml barometric pressure 740 torr temperature of water 24°c termperature of o2 25°c vapor pressure due to water at 25°c 22.4 torr for the conditions listed above, calculate the volume of o2(g) produced at standard conditions of temperature and pressure. (enter your answer in liters)

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
Atrial of this decomposition experiment, using different quantities of reactants than those listed i...
Questions

Health, 16.12.2020 22:40


Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40


Mathematics, 16.12.2020 22:40


History, 16.12.2020 22:40

Physics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40


Chemistry, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40


Mathematics, 16.12.2020 22:40

English, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

Chemistry, 16.12.2020 22:40

 produced at standard conditions of temperature and pressure is 0.2422 L
produced at standard conditions of temperature and pressure is 0.2422 L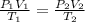
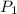 = initial pressure of
= initial pressure of 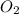 gas = (740-22.4) torr = 717.6 torr
gas = (740-22.4) torr = 717.6 torr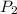 = final pressure of
= final pressure of 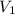 = initial volume of
= initial volume of 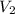 = final volume of
= final volume of 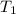 = initial temperature of
= initial temperature of 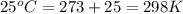
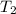 = final temperature of
= final temperature of