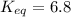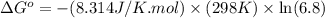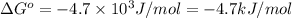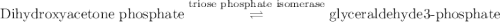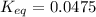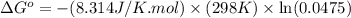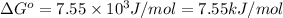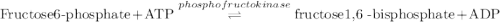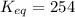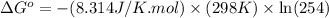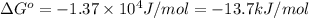Chemistry, 19.11.2019 03:31 joseenrique02
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each of the following metabolically important enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25 °c and ph 7.0. ( a ) glutamate + oxaloacetate aspartate aminotranferase ⇌ aspartate + α -ketoglutarate k ′ eq = 6.8 ( b ) dihydroxyacetone phosphate triose phosphate isomerase ⇌ glyceraldehyde 3 -phosphate k ′ eq = 0.0475 ( c ) fructose 6 -phosphate + atp phosphofructokinase ⇌ fructose 1 , 6 -bisphosphate + adp k ′ eq = 254

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each...
Questions

Mathematics, 24.05.2021 21:10


Mathematics, 24.05.2021 21:10

SAT, 24.05.2021 21:10

Mathematics, 24.05.2021 21:10



History, 24.05.2021 21:10


Mathematics, 24.05.2021 21:10


Physics, 24.05.2021 21:10

Mathematics, 24.05.2021 21:10

Computers and Technology, 24.05.2021 21:10

Mathematics, 24.05.2021 21:10



Mathematics, 24.05.2021 21:10

English, 24.05.2021 21:10

Mathematics, 24.05.2021 21:10

 for the reaction is -4.7 kJ/mol
for the reaction is -4.7 kJ/mol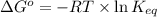
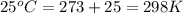
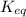 = equilibrium constant
= equilibrium constant