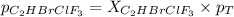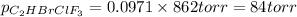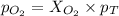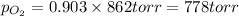Chemistry, 19.11.2019 02:31 marlenemedina247
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has a total pressure of 862 torr. what are the partial pressures of each gas? a. phalothane = 778 torr, po2 = 84 torr b. phalothane = 162 torr, po2 = 700 torr c. phalothane = 84 torr, po2 = 778 torr d. phalothane = 155 torr, po2 = 707 torr e. phalothane = 707 torr, po2 = 155 torr.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

You know the right answer?
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has...
Questions

Physics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40



Advanced Placement (AP), 10.02.2021 23:40

Mathematics, 10.02.2021 23:40



Mathematics, 10.02.2021 23:40




Social Studies, 10.02.2021 23:40


Chemistry, 10.02.2021 23:40



Mathematics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40

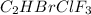 and
and 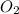 are, 84 torr and 778 torr respectively.
are, 84 torr and 778 torr respectively.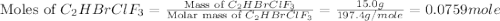
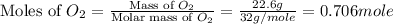
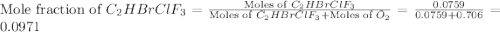
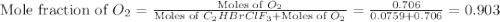
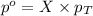
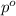 = partial pressure of gas
= partial pressure of gas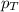 = total pressure of gas
= total pressure of gas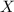 = mole fraction of gas
= mole fraction of gas