
Chemistry, 19.11.2019 02:31 AllyJungkookie
Aspirin (c9h8o4, molecular mass 180.2 g/mol) is synthesized by the reaction of salicylic acid (c7h6o3, molecular mass 138.1 g/mol) with acetic anhydride (c4h6o3, molecular mass 102.1 g/mol) according to the reaction 2 c7h6o3 (s) + c4h6o3(l) → 2c9h8o4(s)+ h2o(l) 2.0g of salicylic acid and 5.4 g of acetic anhydride are mixed and the reaction is allowed to go to completion. do each of the following: a. identify the limiting reagent. b. state how many grams of the excess reagent remain. c. state how many grams of product are produced.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Aspirin (c9h8o4, molecular mass 180.2 g/mol) is synthesized by the reaction of salicylic acid (c7h6o...
Questions


Mathematics, 13.12.2020 07:10





Computers and Technology, 13.12.2020 07:10




Chemistry, 13.12.2020 07:10




Mathematics, 13.12.2020 07:10


Mathematics, 13.12.2020 07:10

Mathematics, 13.12.2020 07:10


Mathematics, 13.12.2020 07:10

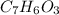 is the limiting reagent.
is the limiting reagent.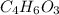 remain.
remain.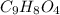 are produced.
are produced.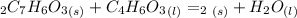
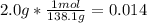
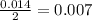
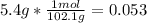
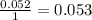
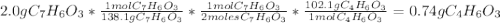 reacts
reacts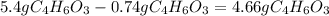 remain
remain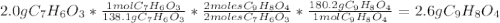 are produced.
are produced.

