Chemistry, 19.11.2019 02:31 locomexicano03
Aflask of volume 2.0 liters, provided with a stopcock, contains oxygen at 20 oc, 1.0 atm (1.013x105 pa). the system is heated to 100 oc with the stopcock open to the atmosphere (like your charles law lab). the stopcock is then closed and the flask cooled to its original temperature (20 oc). k=1.32x10-23 j/k. (a) what is the final pressure of the oxygen in the flask (with the stopcock closed)? (b) how many grams of oxygen remain in the flask?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1


Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Aflask of volume 2.0 liters, provided with a stopcock, contains oxygen at 20 oc, 1.0 atm (1.013x105...
Questions

Health, 08.10.2021 15:00

Mathematics, 08.10.2021 15:00

Mathematics, 08.10.2021 15:00



History, 08.10.2021 15:00

Chemistry, 08.10.2021 15:00

Physics, 08.10.2021 15:00






Biology, 08.10.2021 15:00



Mathematics, 08.10.2021 15:00

Computers and Technology, 08.10.2021 15:00

English, 08.10.2021 15:00

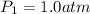
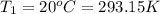
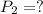
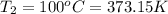
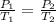
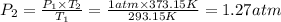
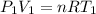 (ideal gas equation)
(ideal gas equation)