Chemistry, 19.11.2019 01:31 EMscary4996
Given the e0 values of the following two half-reactions: zn zn2+ + 2e- e0 = 0.763 volt fe fe2+ + 2e- e0 = 0.441 volt a) write a balanced complete oxidation-reduction reaction? b) explain whether the corrosion of an iron pipe (i. e., fe fe2+) in the presence of zn/zn2+ is possible or not (thermodynamically)? c) explain whether or not zn will protect the corrosion of iron pipe if metallic zn is in contact with the iron pipe?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Given the e0 values of the following two half-reactions: zn zn2+ + 2e- e0 = 0.763 volt fe fe2+...
Questions




Mathematics, 19.03.2020 08:23





English, 19.03.2020 08:23


Engineering, 19.03.2020 08:23


Physics, 19.03.2020 08:24

English, 19.03.2020 08:24

Health, 19.03.2020 08:24





Social Studies, 19.03.2020 08:25

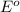 potential will always get reduced and will undergo reduction reaction. Here, zinc will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, zinc will undergo reduction reaction will get reduced.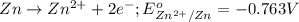
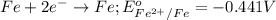
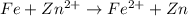
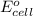 of the reaction, we use the equation:
of the reaction, we use the equation: