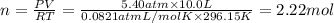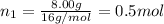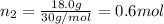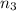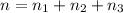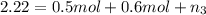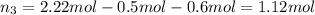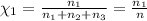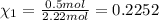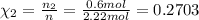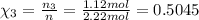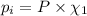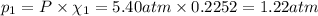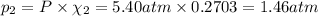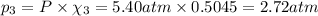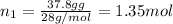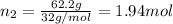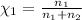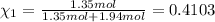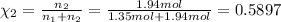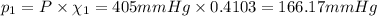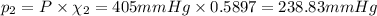Chemistry, 19.11.2019 01:31 kyliexhill
Part a: three gases (8.00 g of methane, ch_4, 18.0g of ethane, c_2h_6, and an unknown amount of propane, c_3h_8) were added to the same 10.0- l container. at 23.0 degrees c, the total pressure in the container is 5.40 atm. calculate the partial pressure of each gas in the container. part b: a gaseous mixture of o_2 and n_2 contains 37.8 % nitrogen by mass. what is the partial pressure of oxygen in the mixture if the total pressure is 405 mmhg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Part a: three gases (8.00 g of methane, ch_4, 18.0g of ethane, c_2h_6, and an unknown amount of pro...
Questions

Computers and Technology, 28.08.2019 16:40

Mathematics, 28.08.2019 16:40








Mathematics, 28.08.2019 16:40


Mathematics, 28.08.2019 16:40


English, 28.08.2019 16:40


Physics, 28.08.2019 16:40


Mathematics, 28.08.2019 16:40


Health, 28.08.2019 16:40