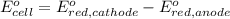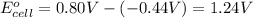Chemistry, 19.11.2019 01:31 burnsmykala23
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given that the standard reduction potential of ag+ to ag (s) is +0.80 v and the standard reduction potential of fe2+ to fe (s) is −0.44 v, calculate the standard cell potential, e°cell.−1.24 v1.24 v2.04 v0.36 v

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1
You know the right answer?
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given...
Questions

Mathematics, 14.02.2020 01:04





Mathematics, 14.02.2020 01:05







History, 14.02.2020 01:05

Mathematics, 14.02.2020 01:05


History, 14.02.2020 01:05

Biology, 14.02.2020 01:05


History, 14.02.2020 01:05

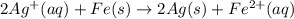
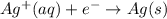
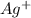 to Ag=
to Ag=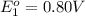
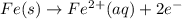
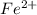 to Fe=
to Fe=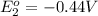
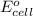 of the reaction, we use the equation:
of the reaction, we use the equation: