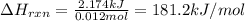Chemistry, 19.11.2019 00:31 keyonaemanieevans
Manganese reacts with hydrochloric acid to produce manganese(ii) chloride and hydrogen gas. mn(s)+2hcl(aq) ? mncl2(aq)+h2(g)
part a when 0.650g mn is combined with enough hydrochloric acid to make 100.0 ml of solution in a coffee-cup calorimeter, all of the mn reacts, raising the temperature of the solution from 23.0? c to 28.2? c. find ? hrxn for the reaction as written. (assume that the specific heat capacity of the solution is 4.18 j/g? c and the density is 1.00 g/ml.) express your answer using three significant figures. ? hrxn = kj

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
Manganese reacts with hydrochloric acid to produce manganese(ii) chloride and hydrogen gas. mn(s)+2h...
Questions

Social Studies, 05.07.2019 07:00



English, 05.07.2019 07:00






English, 05.07.2019 07:00


English, 05.07.2019 07:00

Mathematics, 05.07.2019 07:00


Biology, 05.07.2019 07:10

Biology, 05.07.2019 07:10


Mathematics, 05.07.2019 07:10


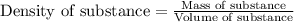
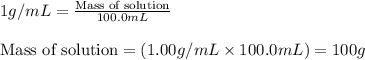
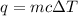
 = change in temperature =
= change in temperature = 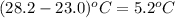
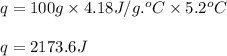
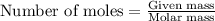
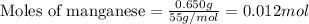
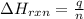
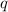 = amount of heat absorbed = 2173.6 J = 2.174 kJ (Conversion used: 1 kJ = 1000 J)
= amount of heat absorbed = 2173.6 J = 2.174 kJ (Conversion used: 1 kJ = 1000 J)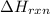 = enthalpy change of the reaction
= enthalpy change of the reaction