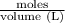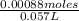A25.0 ml solution of .0600 m edta was added to a 57.0 ml sample containing an unknown concentration of v3+. all v3+ present formed a complex, leaving excess edta in solution.
this solution was back-titrated with a 0440 m ga3+ solution until all the edta reacted requiring 14.0 ml of the ga3+ solution.
what was the original concentration of the v3+ solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A25.0 ml solution of .0600 m edta was added to a 57.0 ml sample containing an unknown concentration...
Questions

Mathematics, 02.02.2020 21:42

History, 02.02.2020 21:42




Social Studies, 02.02.2020 21:42

Biology, 02.02.2020 21:42



Biology, 02.02.2020 21:42


Mathematics, 02.02.2020 21:42


Computers and Technology, 02.02.2020 21:42

Mathematics, 02.02.2020 21:42


Mathematics, 02.02.2020 21:42

Mathematics, 02.02.2020 21:42

Mathematics, 02.02.2020 21:42

Mathematics, 02.02.2020 21:42

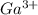 as follows.
as follows.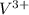 will be as follows.
will be as follows.