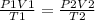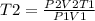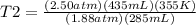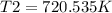Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
Syringe initially holds a sample of gas with a volume of 285 ml at 355 k and 1.88 atm. to what tempe...
Questions


Mathematics, 06.04.2021 05:20



Mathematics, 06.04.2021 05:20



Mathematics, 06.04.2021 05:20





English, 06.04.2021 05:20





English, 06.04.2021 05:20

History, 06.04.2021 05:20

World Languages, 06.04.2021 05:20