
Chemistry, 18.11.2019 20:31 noahdeem135
Industrial production of nitric acid, which is used in many products including fertilizers and explosives, approaches 10 billion kg per year worldwide. the first step in its production is the exothermic oxidation of ammonia, represented by the following equation. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) δh⁰rxn = −902.0 kj if this reaction is carried out using 7.056 ✕ 103 g nh3 as the limiting reactant, what is the change in enthalpy?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
Industrial production of nitric acid, which is used in many products including fertilizers and explo...
Questions

Mathematics, 10.07.2019 20:00


Mathematics, 10.07.2019 20:00




History, 10.07.2019 20:00

Biology, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00

Advanced Placement (AP), 10.07.2019 20:00

Physics, 10.07.2019 20:00



Mathematics, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00


Computers and Technology, 10.07.2019 20:00



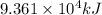
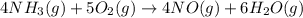
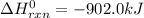
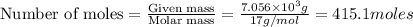
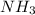 produces = 902.0 kJ of energy
produces = 902.0 kJ of energy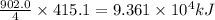 of energy
of energy

