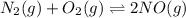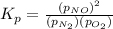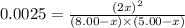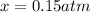Chemistry, 18.11.2019 20:31 sierravick123owr441
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) 2no(g)the equilibrium constant kp for the reaction is 0.0025 at 2127�c. if a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen? a) 0.16 atm b) 0.31 atm c) 3.1 atm d) 7.7 atm e) 7.8 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions

Mathematics, 09.11.2019 04:31

Mathematics, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31


History, 09.11.2019 04:31




Biology, 09.11.2019 04:31

Mathematics, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31

English, 09.11.2019 04:31

English, 09.11.2019 04:31



Mathematics, 09.11.2019 04:31


History, 09.11.2019 04:31

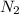 = 8.00 atm
= 8.00 atm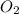 = 5.00 atm
= 5.00 atm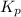 = 0.0025
= 0.0025