
Chemistry, 18.11.2019 19:31 mixedgirlmara
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6 kj / mol rxn. using the following standard enthalpy of formation data, calculate standard enthalpy of formation for sic (s). a. standard enthalpy of formation sio2 (s) = -910.9 kj/mol b. standard enthalpy of formation co (g) = -110.5 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6...
Questions


Mathematics, 23.04.2020 02:06




History, 23.04.2020 02:06

Mathematics, 23.04.2020 02:06


Mathematics, 23.04.2020 02:06




Mathematics, 23.04.2020 02:06


Mathematics, 23.04.2020 02:06


Mathematics, 23.04.2020 02:06


Mathematics, 23.04.2020 02:06

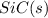 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol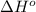
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0379/7554/72c39.png)
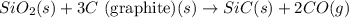
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times \Delta H^o_f_{(CO(g))})]-[(1\times \Delta H^o_f_{(SiO_2(s))})+(3\times \Delta H^o_f_{(C(s))})]](/tpl/images/0379/7554/6a7fe.png)
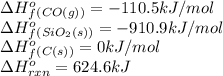
![624.6=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times (-110.5))]-[(1\times (-910.9))+(3\times (0))]\\\\\Delta H^o_f_{(SiC(s))}=-65.3kJ/mol](/tpl/images/0379/7554/c8e18.png)


