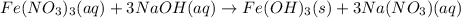Chemistry, 18.11.2019 19:31 meghan0123
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably when a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably a. iron (iii) nitrate. b. sodium hydroxide. c. iron (iii) hydroxide. d. sodium nitrate. e. none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored pr...
Questions







Mathematics, 04.12.2020 17:00





Mathematics, 04.12.2020 17:00






Physics, 04.12.2020 17:00


English, 04.12.2020 17:00