
Chemistry, 16.11.2019 07:31 20jessicar598
The equilibrium constant for the dissolution of silver chloride (agcl(s) ag+(aq) + cl–(aq)) has a value of 1.79 × 10–10. which statement is true?
a. silver chloride is completely soluble in water.
b. silver chloride is essentially insoluble in water.
c. no right choice.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3



Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
The equilibrium constant for the dissolution of silver chloride (agcl(s) ag+(aq) + cl–(aq)) has a va...
Questions

Mathematics, 15.10.2019 08:20

Mathematics, 15.10.2019 08:20

Mathematics, 15.10.2019 08:20


Mathematics, 15.10.2019 08:20

History, 15.10.2019 08:20


Mathematics, 15.10.2019 08:20

Mathematics, 15.10.2019 08:20




History, 15.10.2019 08:20


History, 15.10.2019 08:20




History, 15.10.2019 08:20

 for silver chloride is determined by equilibrium concentrations of dissolved ions. But solubility may vary also at different temperatures. Complete solubility is possible in ammonia solution as it form stable complex as water is not good ligand for Ag+.
for silver chloride is determined by equilibrium concentrations of dissolved ions. But solubility may vary also at different temperatures. Complete solubility is possible in ammonia solution as it form stable complex as water is not good ligand for Ag+. 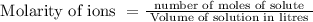
![\left[\mathbf{A} \mathbf{g}^{+}\right]=[\mathbf{C} \mathbf{I}]=\text { molarity of ions }](/tpl/images/0377/2340/63684.png)
![\left[\mathbf{A} \mathbf{g}^{+}\right] \cdot[\mathbf{C} \mathbf{I}]](/tpl/images/0377/2340/66898.png)


