
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrogen gas from the reaction of a sample of sodium hydride with an excess of water fills a volume of 0.475 l above the water. the temperature of the gas is 35 ∘c and the total pressure is 755 mmhg.
find the mass of h2 liberated and the mass of nah that reacted.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2


Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrog...
Questions

Mathematics, 02.12.2020 04:10

Chemistry, 02.12.2020 04:10

Biology, 02.12.2020 04:10


Social Studies, 02.12.2020 04:10

Biology, 02.12.2020 04:10

Advanced Placement (AP), 02.12.2020 04:10



Mathematics, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

History, 02.12.2020 04:10



Mathematics, 02.12.2020 04:10



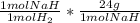 = 0.4480 g NaH
= 0.4480 g NaH

