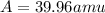Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and the abundances of each of the isotopes: argon36 (35.97 amu; 0.337%), argon-38 (37.96 amu; 0.063%), argon-40 (39.96 amu; 99.600%). 1. 119.89 amu 2. 39.95 amu 3. 39.96 amu 4. 35.97 amu 5. none of these 6. 37.96 amu 7. 37.95 amu 8. 35.96 amu

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

You know the right answer?
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic ma...
Questions




Mathematics, 25.03.2020 21:27



Mathematics, 25.03.2020 21:28


Biology, 25.03.2020 21:28



Social Studies, 25.03.2020 21:28


Mathematics, 25.03.2020 21:28

History, 25.03.2020 21:28

Biology, 25.03.2020 21:28



Mathematics, 25.03.2020 21:28

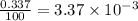
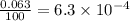
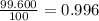
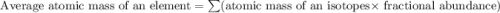
![A=\sum[(35.97\times 3.37\times 10^{-3})+(37.96\times 6.3\times 10^{-4})+(39.96\times 0.996)]](/tpl/images/0377/0178/15521.png)