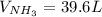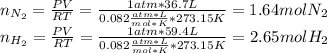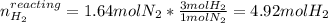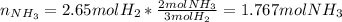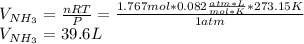Chemistry, 16.11.2019 03:31 kedjenpierrelouis
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh3(g). what volume of ammonia (in l) could be produced by the reaction of 59.4 liters of hydrogen with 36.7 liters of nitrogen at a constant pressure and temperature?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh...
Questions




Mathematics, 12.02.2021 18:50


English, 12.02.2021 18:50


Geography, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50




Mathematics, 12.02.2021 18:50





Social Studies, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50