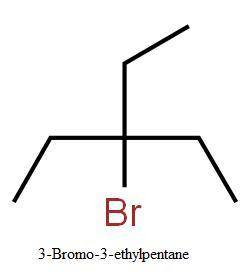
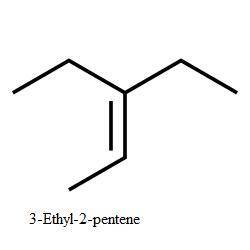
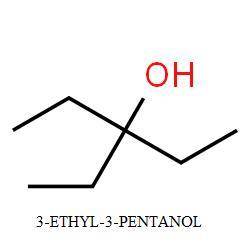
Compound a has molecular formula c7h15br. treatment of compound
a with sodium ethoxide yields only one elimination product (compound
b) and no substitution products. when compound b is treated with dilute sulfuric acid, compound
c is obtained, which has molecular formula c7h16o.
draw the structures of compounds a, b, and c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3


Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Compound a has molecular formula c7h15br. treatment of compound
a with sodium ethoxide yields...
a with sodium ethoxide yields...
Questions

English, 11.09.2019 05:30

Mathematics, 11.09.2019 05:30