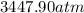Chemistry, 16.11.2019 03:31 prettyluhangel
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by the van der waals equation? the ideal gas law constant is 0.08206 [l•atm] / [mol•k]. for co2, the pressure correction constant is 3.658 l2•atm / mol 2, and the volume correction constant is 0.04286 l / mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1


You know the right answer?
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by th...
Questions



English, 04.02.2021 23:40

English, 04.02.2021 23:40

Social Studies, 04.02.2021 23:40

Computers and Technology, 04.02.2021 23:40

Biology, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40


Mathematics, 04.02.2021 23:40

Physics, 04.02.2021 23:40

Biology, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40


Mathematics, 04.02.2021 23:40



Mathematics, 04.02.2021 23:40

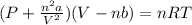
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}][1.0L-(20.0mol\times0.04286 L.mol^{-1} )]=(20.0mol)\times (0.08206L.atm.mol^{-1}.K^{-1})\times (300.0K)](/tpl/images/0376/9858/97073.png)
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}]](/tpl/images/0376/9858/353d5.png) =
=