
Chemistry, 16.11.2019 00:31 samueltaye
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric pressure. b. two moles of ammonia gas are cooled from 325 ? °c to 300 °c at 1.2 atm. c. h2o(l) => h2o(g) at 100 °c at atmospheric pressure. d. caco3(s) => cao(s) + co2(g) at 800 °c at atmospheric pressure. e. co2(s) => co2(g) at atmospheric pressure.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric p...
Questions

Mathematics, 29.11.2020 21:00


History, 29.11.2020 21:00







Mathematics, 29.11.2020 21:00


Mathematics, 29.11.2020 21:00

History, 29.11.2020 21:00

Spanish, 29.11.2020 21:00


English, 29.11.2020 21:00

History, 29.11.2020 21:00



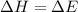
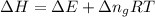
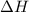 = change in enthalpy
= change in enthalpy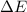 = change in internal energy
= change in internal energy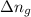 = change in moles
= change in moles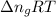 will be zero.
will be zero.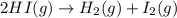 at atmospheric pressure.
at atmospheric pressure.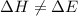
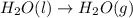 at 100 °C at atmospheric pressure.
at 100 °C at atmospheric pressure.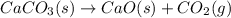 at 800 °C at atmospheric pressure.
at 800 °C at atmospheric pressure.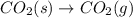 at atmospheric pressure.
at atmospheric pressure.

