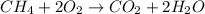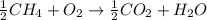Which statement about the following reaction is correct?
ch4 (g) + 2o2 (g) yields co2 (...

Chemistry, 30.01.2020 12:50 dlaskey646
Which statement about the following reaction is correct?
ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o(l) deltah = -890 kj
reacting one mole of oxygen (o2) absorbs 445 kj of energy
reacting one mole of oxygen (o2) releases 445 kj of energy
reacting one mole of methane (ch4) absorbs 890 kj of energy
reacting two moles of methane (ch4) releases 890 kj of energy

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2


Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

You know the right answer?
Questions



English, 07.02.2021 14:00



Mathematics, 07.02.2021 14:00



History, 07.02.2021 14:00





English, 07.02.2021 14:00


Chemistry, 07.02.2021 14:00




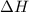 for the reaction comes out to be negative.
for the reaction comes out to be negative.