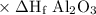Calculate the standard enthalpy of the reaction, δh∘rxn, for the thermite reaction:
2al(s)+fe2o3(s)→2fe(s)+al2o3(s)
elements in their standard state have an enthalpy of formation value of zero. the standard enthalpies of formation of fe2o3 and al2o3 are
δh∘f of fe2o3(s)=−825.5 kj/molδh∘f of al2o3(s)=−1675 kj/mol
express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Calculate the standard enthalpy of the reaction, δh∘rxn, for the thermite reaction:
2al(s)+fe...
2al(s)+fe...
Questions

History, 22.09.2019 16:10

Mathematics, 22.09.2019 16:20

Biology, 22.09.2019 16:20


History, 22.09.2019 16:20

Mathematics, 22.09.2019 16:20


History, 22.09.2019 16:20

Mathematics, 22.09.2019 16:20


World Languages, 22.09.2019 16:20

Social Studies, 22.09.2019 16:20



Chemistry, 22.09.2019 16:20

History, 22.09.2019 16:20


Mathematics, 22.09.2019 16:20

Health, 22.09.2019 16:20

Mathematics, 22.09.2019 16:20

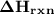 ) can be given by:
) can be given by:
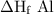 + 1
+ 1 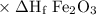
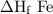 + 1
+ 1