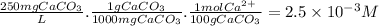The buffer solution is used to control the ph to insure that it does not become too high because excessively basic solutions could cause the corresponding hydroxides of hard metal ions (such as ca(oh)2 and mg(oh)2) to precipitate. using the calcium ion as a typical representative, just how high a ph do you think could be considered as "too high" for a solution with a hardness of about 250 ppm caco3? ksp for ca(oh)2 is 6.5 x 10-6.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

You know the right answer?
The buffer solution is used to control the ph to insure that it does not become too high because exc...
Questions

Mathematics, 10.10.2020 14:01


Health, 10.10.2020 14:01


Mathematics, 10.10.2020 14:01

Chemistry, 10.10.2020 14:01



History, 10.10.2020 14:01


Mathematics, 10.10.2020 14:01


English, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01

Physics, 10.10.2020 14:01



Physics, 10.10.2020 14:01

History, 10.10.2020 14:01