Chemistry, 15.11.2019 20:31 Mrblunt5613
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g) δh o rxn = −1255.8 kj given δh o f of co2(g) = −393.5 kj/mol and δh o f of h2o(g) = −241.8 kj/mol, find δh o f of c2h2(g).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g)...
Questions

Biology, 16.12.2021 01:00

English, 16.12.2021 01:00

Business, 16.12.2021 01:00

Social Studies, 16.12.2021 01:00


Mathematics, 16.12.2021 01:00


Biology, 16.12.2021 01:00


English, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00




Physics, 16.12.2021 01:00

Mathematics, 16.12.2021 01:00




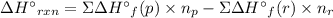
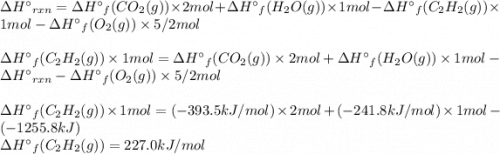
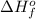 , of C₂H₂ is 227 kJ/mol
, of C₂H₂ is 227 kJ/mol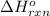 = −1255.8 kJ
= −1255.8 kJ