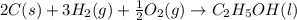Chemistry, 15.11.2019 20:31 johnnybones03
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that represents the formation of liquid ethanol? a. 2 c(s) + 6 h(g) + o(g) → c2h5oh(l) b. 2 c(s) + 3 h2(g) + ½ o2(g) → c2h5oh(l) c. 2co2(g) + 3h2o(g) → c2h5oh(l) + 3 o2(g) d. 4 c(s) + 6 h2(g) + o2(g) → 2 c2h5oh(l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that rep...
Questions


Business, 31.12.2020 01:00





Health, 31.12.2020 01:00




Business, 31.12.2020 01:00

Mathematics, 31.12.2020 01:00



Social Studies, 31.12.2020 01:00